90 Days Effective
For Personal Use
Defense Against Pathogens
Clinically Tested
90 Days Effective
For Personal Use
Defense Against Pathogens
Clinically Tested
Designed for everyday use,
providing an extra layer of defense against viruses, bacteria and spores.
Patented,
clinically tested,
up to 90 days of air disinfecting around you.
Clinically tested
Effective against Corona
Patented
To be used in closed and crowded spaces.
It can not only help to protect you but others as well.
The disinfectant is constantly released,
its effectiveness travels and spreads along with you.
It is easy and safe to use and provides an extra layer of defense to your everyday.
MyAirShield™ is the first portable air disinfectant that is clinically tested and approved, including for efficacy against Corona variants.
The MyAirShield agent is tested by multiple third-party laboratories for its efficacy against pathogens like viruses, bacteria, and spores, as well as for being harmless in an everyday usage.
Our patented pearls are designed to slowly release a controlled, low concentration of the disinfectant, aiding an atmospheric shield of additional defense around you.
Efficient and safe to use!
WATCH THE PRODUCT VIDEO

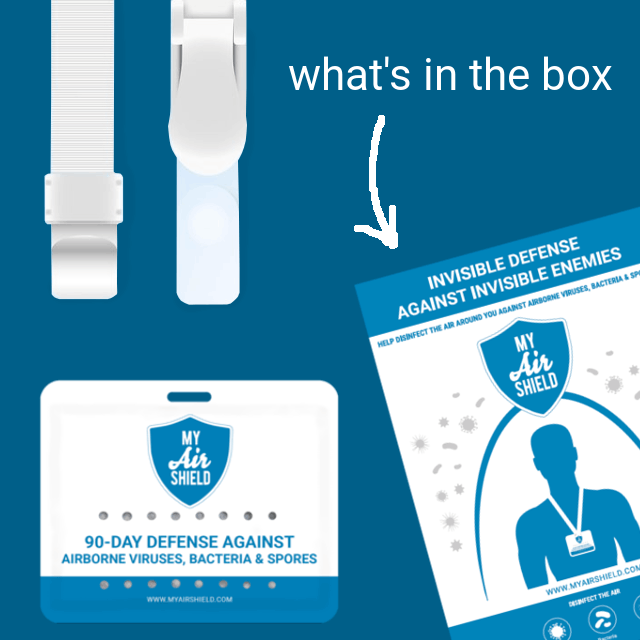
The MyAirShield Badge is an advanced device that aids in disinfecting the air around you by spreading an atmospheric shield of the MyAirShield disinfectant, one of the best agents against microbial life. Each pouch contains our patented encapsulated Pearls designed to slowly release a controlled, low concentration of the disinfectant for 90 days.
Open the first layer of packaging. The second package contained within it is the wearable MyAirShield™ badge, which contains encapsulated pearls. Do not damage, tear, or open the card. Attach the card to your clothes, bag, or around your neck, or in any area where you would prefer a cleaner and disinfected breathing space, making sure that the aeration holes face away from the surface that it is attached to, to ensure maximum air flow and efficacy.
Once the sealed package is opened, the release of the agent will start immediately - the batch is activated immediately. After 90 days, discard the card in a safe and environmentally friendly manner.
The MyAirShield disinfectant has been proven as an effective: disinfectant, sanitizer, virucide, fungicide, algaecide, slimicide, and deodorizer of spores among other things. It has a wide range of applications and has been used for many years in various industries.
SUGGESTED USE: In closed spaces, homes, hospitals, offices, classrooms, conferences, arenas, cinemas, cafes and restaurants, libraries, while shopping, and while traveling on cruise liners, trains, planes, cars, buses, metros, etc.
MyAirShield Pearls© are made up of a mixture of solid components. By the passage of ambient air through them they naturally interact with other components in the air, like the CO2 and humidity, causing the generation of the MyAirShield agent. Its lattice structure allows for the slow release of the agent into the surrounding atmosphere.
The unique antimicrobial properties of the MyAirShield agent were tested on different microorganisms including, bacteria, fungus and viruses.
Due to its small size, the agent is able to penetrate into micro-organisms and to react with pathogens structure, causing them to break down. Human cells are much larger than micro-organisms such as viruses and bacteria which makes them resistant to the MyAirShield agent in its intended use, hence human cells do not get damaged.
Upon absorption of the MyAirShield agent the human cells have the advantage of being able to break down its structure into ions which the body uses for everyday physiological processes in turn preventing its accumulation inside of the cell and thus avoiding damage.
The product is produced in compliance with ISO9001 and ISO14001.
Tests show that the MyAirShield agent is highly effective against a large variety of airborne pathogens, such as viruses, bacteria and spores. These tests include those for release rate, toxicity and efficiency - including effectiveness against corona variants. MyAirShield collaborates with well known laboratories to ensure broad and profound testing to meet international standards. Additionally, independent third-party studies confirm its efficiency.
The MyAirShield testing include studies for release rates and toxicity, proving the MyAirShield Badge to be safe and effective to use every day. It is i.a. ECHA (EU) registered, COFEPRIS certified and FDA (US) registered as a Class 1 Medical Device. It may be used in closed and crowded spaces, including hospitals, on public transport, cafés and restaurants, in school, in the office and other public spaces, and even in your own home. It can be used as an additional disinfectant measure for office staff, elderly people, children and the public in general. The MyAirShield Badge is secure to wear due to its safety lock and the adjustable lanyard.
The following test studies have been conducted and are ongoing at multiple third-pary laboratories:
• Efficiency Tests
Proving the efficiency of the MyAirShield agent against common pathogens like viruses, bacteria, spores.
Standards i.a.: WS/T 206
• Release Tests
Proving the maximum release values of CLO2 from the MyAirShield Batch are compliant with legal limits, thus unharmful for humans.
Standards i.a.: ISO/IEC17025:2005
• Toxicity Tests
Proving that the MyAirShield agent is not harmful for humans, proven in each an oral, inhalation and dermal test.
Standards i.a.: OECD420, OECD431, EPI-200-SCT
• Corona Efficiency Test
Proving the efficiency of the MyAirShield disinfectant against Corona virus variants.
Tested Pathogens
Tested on most standard pathogens as i.a.
• Aspergillus
• Adenovirus
• B.Pertussis
• C.Pneumoniae
• CoV_NL63
• CoV_OC43
• L.Pneumophila
• M.Pneumoniae
• mecA
• Rhinovirus
• S.Agalactiae
• S.Aureus
• S.Pneumoniae
• Covid-19
• Psedomonia
• EColi
• Candida
ECHA registered
COFEPRIS certified
FDA registered as a Class 1 Medical Device
Europe
Luxembourg, Germany, France, Denmark, Sweden, Norway, Finland, Poland, United Kingdom, Kazakhstan, Greece, Bulgaria, Hungary, Austria, Czech Republic, Serbia, Ireland, Estonia, Lithuania, Latvia, Croatia, Bosnia and Herzegovina, Slovakia, Netherlands, Belgium, Albania, North Macedonia, Turkey, Slovenia, Montenegro, Azerbaijan, Cyprus, Monaco
North America
Canada, United States, Mexico
Central America
Belize, El Salvador, Guatemala, Honduras, Nicaragua, Panama
South America
Argentina, Brazil, Chile, Colombia, Ecuador, Peru, Uruguay
Asia
China, India, Pakistan, Bangladesh, Turkey, Uzbekistan, Saudi Arabia, Kazakhstan, Azerbaijan, United Arab Emirates, Israel, Hong Kong
Afrika
Nigeria, Ethiopia, South Africa, Kenya, Uganda, Ghana, Cameroon, Niger, Zambia, Rwanda, Liberia, Central African Republic, Namibia, Gambia, Lesotho, Djibouti
Note: for some countries the official registration is pending and expected to be granted soon. In case of any question, please contact us.